How Gut Inflammation May Trigger Brain Disorders
In a major advance for microbiota and neuroimmunology research, a new study led by Sano Teruyuki reveals a mechanistic link between intestinal inflammation and central nervous system (CNS) pathology.
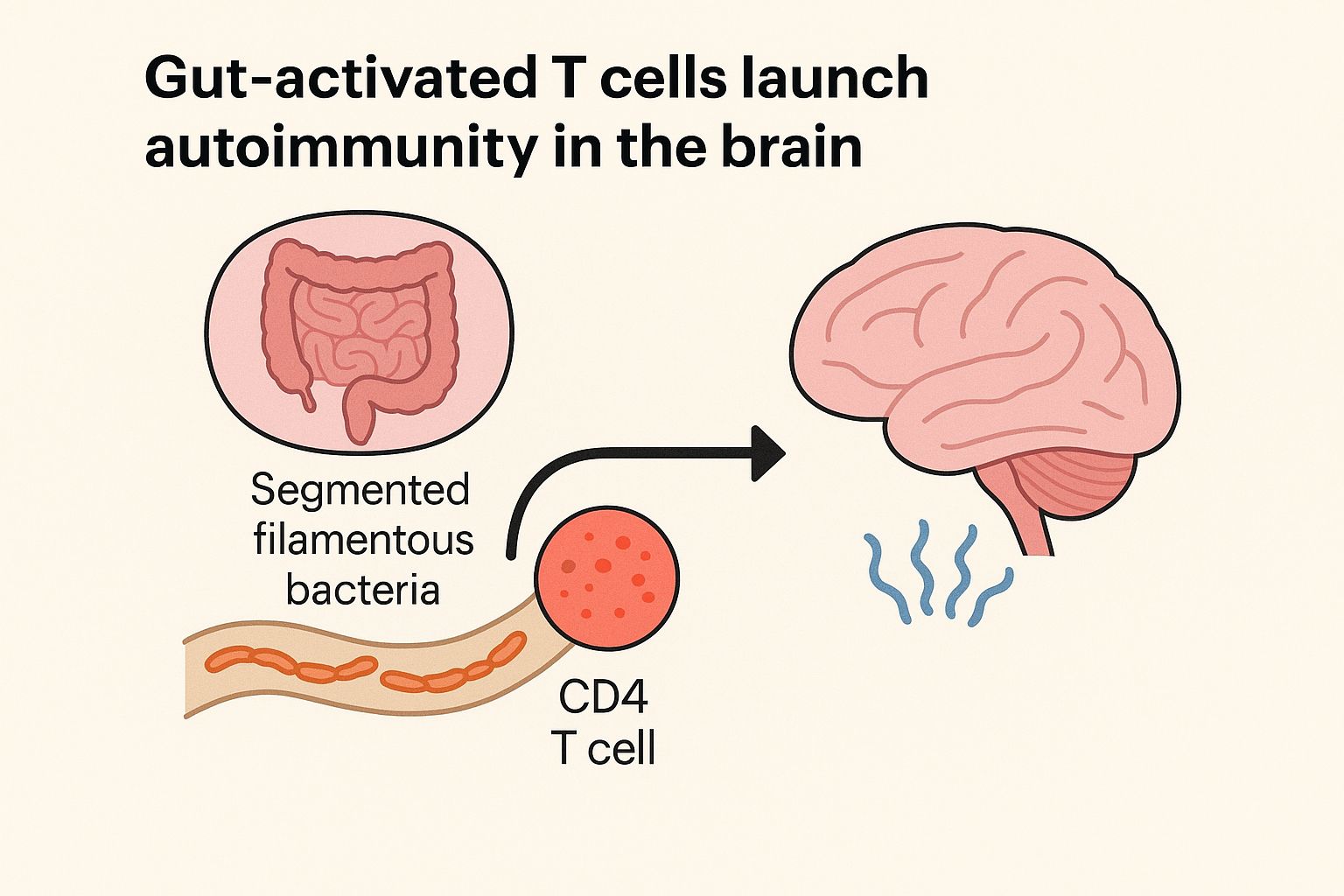
While the gut microbiome has long been implicated in diseases ranging from IBD to neurodegeneration, this study demonstrates how gut-primed CD4⁺ T cells, trained to recognize segmented filamentous bacteria (SFB), can infiltrate the CNS—a normally sterile environment—and initiate neuroinflammation.
Key mechanistic insights:
- Microbiota-specific CD4⁺ T cells (T_comm) activated in the inflamed gut acquire the capacity to enter the CNS.
- These T cells can be re-stimulated in the brain via molecular mimicry, misidentifying host-derived antigens as microbial.
- Upon reactivation, they secrete high levels of GM-CSF, IFNγ, and IL-17A, which drive microglial activation and CNS inflammation.
- Impaired immune regulation—such as loss of regulatory T cells—facilitates this pathogenic migration.
- Immune checkpoint blockade therapies (e.g., anti-PD-1/CTLA-4) exacerbate this process, suggesting a mechanism for neurotoxic side effects observed in some cancer patients.
This work highlights a gut–brain–immune axis with major implications for understanding and managing immune and neuroinflammatory diseases.


