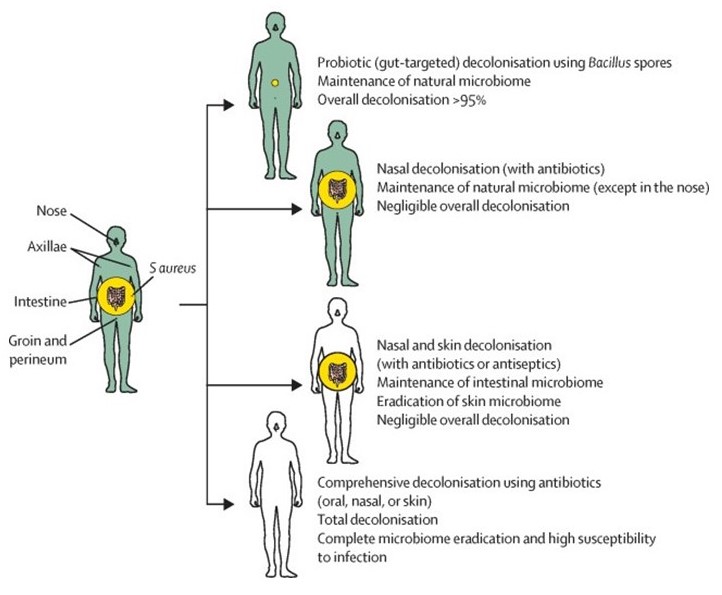
 S aureus distribution in the human body and comparison of decolonisation strategies.
S aureus distribution in the human body and comparison of decolonisation strategies.
Decolonisation is considered a valuable means to reduce Staphylococcus aureus infection rates. However, previous topical strategies targeting the nose or skin had little success, and oral antibiotic-based decolonisation is ill advised because of eradication of the microbiota and development of antibiotic resistance.
Michael Otto and colleagues from the National Institute of Allergy and Infectious Diseases, previously showed that the probiotic Bacillus subtilis significantly diminished S aureus at the main intestinal colonisation site via specific bacterial interaction in mice; in this study, they tested this probiotic approach to control S aureus colonisation in humans.
Dr. Otto and his team did a single-centre, phase 2, double-blind, randomised, placebo-controlled trial in adults from the Songkhla region of Thailand who were colonised by S aureus. Eligible participants were adults (aged ≥18 years) without history of intestinal disease, antibiotic treatment, or hospital admission within the previous 90 days. Participants were excluded if they were pregnant, breastfeeding, taking probiotics, or had diarrhoea.
Participants were allocated to groups by computer randomisation in blocks of four, and research coordinators were masked to group allocation. Participants received 250 mg of probiotic B subtilis MB40 or placebo once per day for 30 days and S aureus colonisation was determined after the last dose was received. The primary outcome was colonisation by S aureus in the intestine and nares after intervention.
Results showed that 115 participants were colonised by S aureus, either in the intestine, nose, or both, and were randomly assigned to treatment and placebo groups. Oral probiotic B subtilis resulted in significant reduction of S aureus in stool and nose. There were no differences in adverse effects or significant microbiome changes between the intervention and placebo groups.
In summary, B subtilis probiotic eliminated more than 95% of the total S aureus colonising the human body without altering the microbiota. This probiotic strategy offers several key advantages over presently used decolonisation strategies for potential use in people with chronic or long-term risk of S aureus infection. Furthermore, by establishing a defining role of the intestinal colonisation site, these findings call for revisiting fundamental notions about S aureus colonisation.
Targeting Microbiota 2023 will keep you updated with the latest probiotic discoveries and research. A full session will be dedicated to innovations. You can submit your latest research on microbiota here.
Article DOI.
Image Credits: Piewngam et al. The Lancet Microbe (2023)
Media contact:
International Society of Microbiota
[email protected]
Targeting Microbiota 2023 Congress
October 17-19, 2023
Website | LinkedIn | Facebook
 S aureus distribution in the human body and comparison of decolonisation strategies.
S aureus distribution in the human body and comparison of decolonisation strategies.